(Bevacizumab)
Anti-VEGF Recombinant Monoclonal Antibody
Indication : Avegra®
Metastatic carcinoma of the colon or rectum (mCRC)
• Avegra®, in combination with a fluoropyrimidine-based chemotherapy is indicated for treatment of adult patients with metastatic carcinoma of the colon or rectum.
Non-small cell lung cancer other than predominantly squamous cell history (NSCLC)
First-line treatment of non-squamous NSCLC in combination with platinum-based chemotherapy
• Avegra® is administered for adult patients with unresectable advanced, metastatic or recurrent non-small cell lung cancer other than predominantly squamous cell histology.
First-line treatment of non-squamous NSCLC with EGFR activating mutations in combination with erlotinib
• Avegra® in combination with erlotinib, for adult patients with unresectable advanced, metastatic or recurrent non-squamous non-small cell lung cancer with
Epidermal Growth Factor Receptor (EGFR) activating mutations.
Epithelial ovarian, fallopian tube, or primary peritoneal cancer
Front-line treatment:
• Avegra® in combination with carboplatin and paclitaxel for adult patients with advanced (International Federation of Gynecology and Obstetrics (FIGO) stage IIIB,
IIIC and IV) epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Treatment of platinum-sensitive recurrent disease:
• Avegra® in combination with carboplatin and gemcitabine for adult patients, who have not received prior therapy with bevacizumab or other VEGR inhibitors or VEGR receptor-targeted agents, for 6 cycles and up to 10 cycles follow by continued use of Avegra as single agent until disease progression.
Treatment of platinum-resistant recurrent disease:
• Avegra® in combination with paclitaxel, topotecan or pegylated liposomal doxorubicin indicated for the treatment of adult patients with platinum-resistant
recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who received no more than two prior chemotherapy regimens and who have not received
prior therapy with bevacizumab or other VEGF inhibitors or VEGF receptor-targeted agents.
Carcinoma of the cervix
• Avegra® in combination with paclitaxel+cisplatin or alternatively, paclitaxel and topotecan in patients who cannot receive platinum therapy, is indicated for the treatment of adult patients with persistant, recurrent, or metastatic carcinoma of the cervix.
Renal cell cancer
• Avegra® in combination with interferon alfa-2a is indicated for first line treatment of adult patients with advanced and/or metastatic renal cell cancer.
Glioblastoma
• As monotherapy.
ใช้เฉพาะโรงพยาบาล
ความถูกต้องของโฆษณานี้ เป็นความรับผิดชอบของผู้โฆษณา มิได้ดำเนินการโดยสำนักงานคณะกรรมการอาหารและยา
เลขทะเบียน 1C 2/66 (NBS)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ.2-1062/2566
Metastatic carcinoma of the colon or rectum (mCRC)
• Avegra®, in combination with a fluoropyrimidine-based chemotherapy is indicated for treatment of adult patients with metastatic carcinoma of the colon or rectum.
Non-small cell lung cancer other than predominantly squamous cell history (NSCLC)
First-line treatment of non-squamous NSCLC in combination with platinum-based chemotherapy
• Avegra® is administered for adult patients with unresectable advanced, metastatic or recurrent non-small cell lung cancer other than predominantly squamous cell histology.
First-line treatment of non-squamous NSCLC with EGFR activating mutations in combination with erlotinib
• Avegra® in combination with erlotinib, for adult patients with unresectable advanced, metastatic or recurrent non-squamous non-small cell lung cancer with
Epidermal Growth Factor Receptor (EGFR) activating mutations.
Epithelial ovarian, fallopian tube, or primary peritoneal cancer
Front-line treatment:
• Avegra® in combination with carboplatin and paclitaxel for adult patients with advanced (International Federation of Gynecology and Obstetrics (FIGO) stage IIIB,
IIIC and IV) epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Treatment of platinum-sensitive recurrent disease:
• Avegra® in combination with carboplatin and gemcitabine for adult patients, who have not received prior therapy with bevacizumab or other VEGR inhibitors or VEGR receptor-targeted agents, for 6 cycles and up to 10 cycles follow by continued use of Avegra as single agent until disease progression.
Treatment of platinum-resistant recurrent disease:
• Avegra® in combination with paclitaxel, topotecan or pegylated liposomal doxorubicin indicated for the treatment of adult patients with platinum-resistant
recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who received no more than two prior chemotherapy regimens and who have not received
prior therapy with bevacizumab or other VEGF inhibitors or VEGF receptor-targeted agents.
Carcinoma of the cervix
• Avegra® in combination with paclitaxel+cisplatin or alternatively, paclitaxel and topotecan in patients who cannot receive platinum therapy, is indicated for the treatment of adult patients with persistant, recurrent, or metastatic carcinoma of the cervix.
Renal cell cancer
• Avegra® in combination with interferon alfa-2a is indicated for first line treatment of adult patients with advanced and/or metastatic renal cell cancer.
Glioblastoma
• As monotherapy.
ใช้เฉพาะโรงพยาบาล
ความถูกต้องของโฆษณานี้ เป็นความรับผิดชอบของผู้โฆษณา มิได้ดำเนินการโดยสำนักงานคณะกรรมการอาหารและยา
เลขทะเบียน 1C 2/66 (NBS)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ.2-1062/2566
อ่านเพิ่มเติม
(Equine antirabies immunoglobulin fragments)
Presentation:
Vials containing 1000 I.U (5 mL).
Vials containing 400 I.U (2 mL).
Indication : VINRAB provides passive immunization against rabies for prevention of rabies in patients at risk of being exposed to rabies after contact with a rabid animal or an animal presumed to be rabid. Antirabies serum itself does not constitute an antirabies treatment and should always be used in conjunction with rabies vaccine.
เลขทะเบียน 1C 24/55 (B)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ.2-18/2566
เลขทะเบียน 1C 24/55 (B)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ.2-18/2566
อ่านเพิ่มเติม
(Enzyme refined, Equine Diphtheria antitoxic immunoglobulin fragments)
Indication : Diphtheria antitoxin has been used to provide passive immunity against Diphtheria.
เลขทะเบียน 1C 1/60 (B)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ. 1122/ 2563
เลขทะเบียน 1C 1/60 (B)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ. 1122/ 2563
อ่านเพิ่มเติม
(Human tetanus immunoglobulin)
Indication : 1. Post-exposure prophylaxis
Immediate prophylaxis after tetanus prone injuries in patients not adequately vaccinated, in patients whose immunisation status is not known with certainty, and in patients with severe deficiency in antibody production.
2. Therapy of clinically manifest tetanus
Active tetanus vaccination should always be administered in conjunction with tetanus immunoglobulin unless there are contraindications or confirmation of adequate vaccination.
เลขทะเบียน 1C 50/52 (B)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ. 1177/ 2563
Immediate prophylaxis after tetanus prone injuries in patients not adequately vaccinated, in patients whose immunisation status is not known with certainty, and in patients with severe deficiency in antibody production.
2. Therapy of clinically manifest tetanus
Active tetanus vaccination should always be administered in conjunction with tetanus immunoglobulin unless there are contraindications or confirmation of adequate vaccination.
เลขทะเบียน 1C 50/52 (B)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ. 1177/ 2563
อ่านเพิ่มเติม
(Recombinant Human Epidermal Growth Factor )
Indication : - For topical healing of neuropathic diabetic foot ulcers, bedsores (pressure ulcers) and chronic leg ulcers.
เลขทะเบียน 1C 5/52 (NB)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ. 1117/ 2563
เลขทะเบียน 1C 5/52 (NB)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ. 1117/ 2563
อ่านเพิ่มเติม
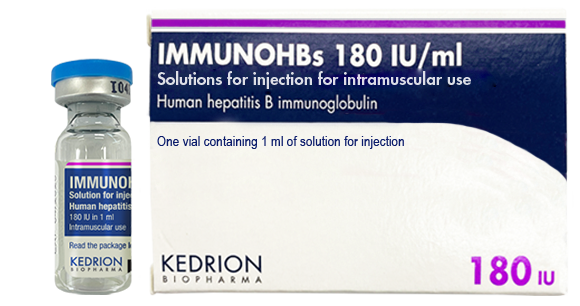
(Human hepatitis B immunoglobulin)
Indication : • Prevention of hepatitis B virus re-infection after liver transplantation for hepatitis B induced liver failure;
• Immunoprophylaxis of hepatitis B
- In case of accidental exposure in non-immunised subjects (including persons whose vaccination is incomplete or status unknown)
- In haemodialysed patients, until vaccination has become effective
- In the newborn of a hepatitis B virus carrier-mother
- In subjects who did not show an immune response (no measurable hepatitis B antibodies) after vaccination and for whom a continuous prevention is necessary due to the continuous risk of being infected with hepatitis B.
ความถูกต้องของโฆษณานี้เป็นความรับผิดชอบของผู้โฆษณา มิได้ดำเนินการโดยสำนักงานคณะกรรมการอาหารและยา
เลขทะเบียน 1C 251/50
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ.2-399/2566
• Immunoprophylaxis of hepatitis B
- In case of accidental exposure in non-immunised subjects (including persons whose vaccination is incomplete or status unknown)
- In haemodialysed patients, until vaccination has become effective
- In the newborn of a hepatitis B virus carrier-mother
- In subjects who did not show an immune response (no measurable hepatitis B antibodies) after vaccination and for whom a continuous prevention is necessary due to the continuous risk of being infected with hepatitis B.
ความถูกต้องของโฆษณานี้เป็นความรับผิดชอบของผู้โฆษณา มิได้ดำเนินการโดยสำนักงานคณะกรรมการอาหารและยา
เลขทะเบียน 1C 251/50
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ.2-399/2566
อ่านเพิ่มเติม
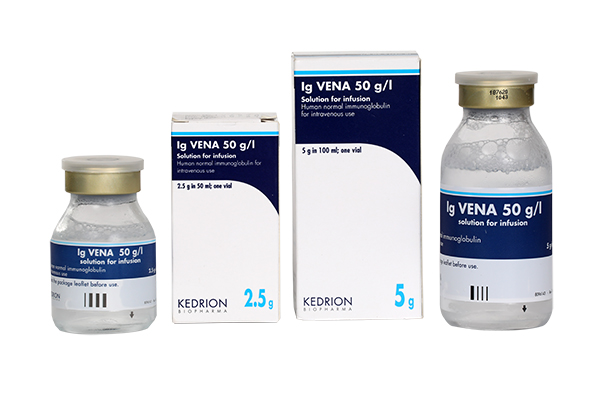
(Human normal immunoglobulin (IgIV))
Indication : Replacement therapy in adults, and children and adolescents (0-18 years) in:
• Primary immunodeficiency syndromes with impaired antibody production.
• Hypogammaglobulinaemia and recurrent bacterial infections in patients with chronic lymphocytic leukaemia, in whom prophylactic antibiotics have failed.
• Hypogammaglobulinaemia and recurrent bacterial infections in plateau phase multiple myeloma patients who have failed to respond to pneumococcal immunisation.
• Hypogammaglobulinaemia in patients after allogeneic haematopoietic stem cell transplantation (HSCT).
• Congenital AIDS with recurrent bacterial infections.
Immunomodulation in adults, and children and adolescents (0-18 years) in:
• Primary immune thrombocytopenia (ITP), in patients at high risk of bleeding or prior to surgery to correct the platelet count.
• Guillain Barré syndrome.
• Chronic Inflammatory Demyelinating Poliradiculoneuropathy (CIDP).
• Kawasaki disease.
เลขทะเบียน 1C 30/49
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ. 1181/ 2563
• Primary immunodeficiency syndromes with impaired antibody production.
• Hypogammaglobulinaemia and recurrent bacterial infections in patients with chronic lymphocytic leukaemia, in whom prophylactic antibiotics have failed.
• Hypogammaglobulinaemia and recurrent bacterial infections in plateau phase multiple myeloma patients who have failed to respond to pneumococcal immunisation.
• Hypogammaglobulinaemia in patients after allogeneic haematopoietic stem cell transplantation (HSCT).
• Congenital AIDS with recurrent bacterial infections.
Immunomodulation in adults, and children and adolescents (0-18 years) in:
• Primary immune thrombocytopenia (ITP), in patients at high risk of bleeding or prior to surgery to correct the platelet count.
• Guillain Barré syndrome.
• Chronic Inflammatory Demyelinating Poliradiculoneuropathy (CIDP).
• Kawasaki disease.
เลขทะเบียน 1C 30/49
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ. 1181/ 2563
อ่านเพิ่มเติม
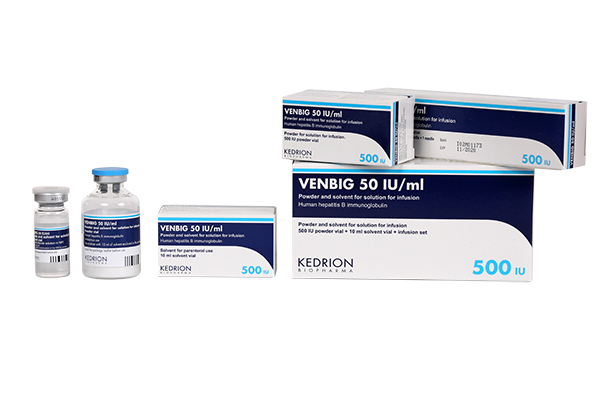
(Human hepatitis B immunoglobulin)
Indication : • Prevention of hepatitis B virus recurrence after liver transplantation for hepatitis B virus induced liver failure in combination with antiviral therapy.
• Immunoprophylaxis of hepatitis B:
- In case of accidental exposure in non-immunised subjects (including persons whose vaccination is incomplete or status unknown)
- In haemodialysed patients, until vaccination has become effective
- In the newborn of a hepatitis B virus carrier-mother
- In subjects who did not show an immune response (no measurable hepatitis B antibodies) after vaccination and for whom a continuous prevention is necessary due to the continuous risk of being infected with hepatitis B.
เลขทะเบียน 1C 33/54 (NBC)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ. 1184/ 2563
 เป็นยาใหม่ ใช้เฉพาะสถานพยาบาลแพทย์ควรติดตามผลการใช้ยา
เป็นยาใหม่ ใช้เฉพาะสถานพยาบาลแพทย์ควรติดตามผลการใช้ยา
• Immunoprophylaxis of hepatitis B:
- In case of accidental exposure in non-immunised subjects (including persons whose vaccination is incomplete or status unknown)
- In haemodialysed patients, until vaccination has become effective
- In the newborn of a hepatitis B virus carrier-mother
- In subjects who did not show an immune response (no measurable hepatitis B antibodies) after vaccination and for whom a continuous prevention is necessary due to the continuous risk of being infected with hepatitis B.
เลขทะเบียน 1C 33/54 (NBC)
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ. 1184/ 2563
 เป็นยาใหม่ ใช้เฉพาะสถานพยาบาลแพทย์ควรติดตามผลการใช้ยา
เป็นยาใหม่ ใช้เฉพาะสถานพยาบาลแพทย์ควรติดตามผลการใช้ยา
อ่านเพิ่มเติม
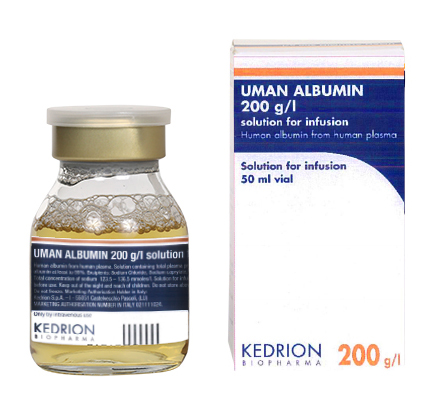
(total plasma proteins to 20%, of which human albumin at least to 95%.)
Indication : Restoration and maintenance of circulating blood volume where volume deficiency has been demonstrated, and use of a colloid is appropriate.
The choice of albumin rather than artificial colloid will depend on the clinical situation of the individual patient, based on official recommendations.
ความถูกต้องของโฆษณานี้เป็นความรับผิดชอบของผู้โฆษณา มิได้ดำเนินการโดยสำนักงานคณะกรรมการอาหารและยา
เลขทะเบียน 1C 99/46
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ.2-384/2566
The choice of albumin rather than artificial colloid will depend on the clinical situation of the individual patient, based on official recommendations.
ความถูกต้องของโฆษณานี้เป็นความรับผิดชอบของผู้โฆษณา มิได้ดำเนินการโดยสำนักงานคณะกรรมการอาหารและยา
เลขทะเบียน 1C 99/46
โปรดอ่านรายละเอียดเพิ่มเติมในเอกสารกำกับยา
ฆศ.2-384/2566
อ่านเพิ่มเติม